
Raleigh Molecular is widely recognized as a leader in providing custom Conjugation Services, which serve as valuable tools for Drug Delivery, Targeted Drug Delivery, Synergistic Drug and Gene Delivery, or Diagnostic applications.
Raleigh Molecular’s technical experts seamlessly integrate with your drug development team to provide tailored design and synthesis solutions for conjugating small molecules like drugs, metabolites, and labeled compounds with synthetic or natural polymers for targeted applications. Leveraging specialized analytical tools, our scientists develop precise analytical methods to characterize your drug conjugates accurately.
Conjugation involves covalently linking two molecules, polymers, biomolecules, or biopolymers together. Conjugating small molecule therapeutics to polymers can enhance efficacy by improving pharmacokinetic and pharmacodynamic properties through mechanisms such as reducing plasma protein binding, decreasing toxicity, or enhancing stability.
Drug conjugates are currently under development for various therapeutic indications, including cardiovascular disease, acute invasive fungal infections, immune modulation, and more. Conjugation is also being explored as a strategy for synergistic drug and gene delivery by linking a drug with a cationic polymer like PEI, which can complex with negatively charged DNA. These polyplexes release the DNA and drug molecule upon internalization into the cell, offering potential benefits in combined drug and gene delivery approaches.
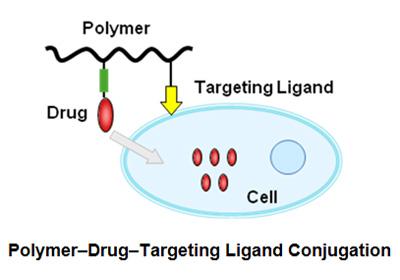
Ongoing research is focused on developing new drug delivery systems for targeted drug delivery. Incorporating target cell-specific ligands such as EGF and RGD peptides into polymer-drug conjugates holds promise for achieving selective and targeted drug delivery solutions.
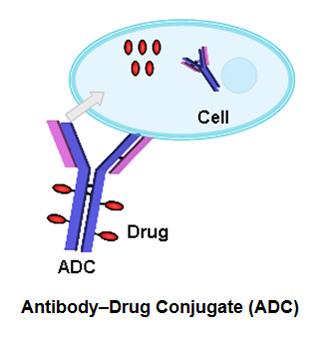
The most prominent class of conjugated drug products in development is the antibody-drug conjugate (ADC). These conjugates combine a drug with a monoclonal antibody, enabling selective targeting to specific cells or tissues. The drug component may be released through enzymatic cleavage of the linker connecting the antibody and drug molecule. ADCs represent a significant advancement in targeted drug delivery strategies.
Researchers are strategically exploring the conjugation approach for targeted imaging to achieve significant contrast enhancement at diseased sites. Conjugating a targeting ligand such as RGD or EPR with a radio-ligand chelator like DOTA to a polymer has introduced a new dimension to diagnostic imaging techniques such as PET and MRI for precise targeted tumor mapping. This approach holds promise for improving diagnostic accuracy and treatment monitoring in oncology and other disease areas.
Raleigh Molecular possesses the necessary analytical tools and expertise crucial for the field of drug conjugation. Meeting the analytical requirements for supporting the manufacturing, testing, as well as regulatory safety and efficacy assessments of drug conjugates is technically demanding.
In drug conjugation, the biological or polymeric carrier can be linked to the drug via site-specific linking or through random linking involving multiple sites with similar reactivity. Stoichiometry, representing the mean ratio of the carrier to the drug, is a relevant measure for characterizing the conjugate in the case of site-specific linking. However, in instances of random linking, the product lacks homogeneity, and the mean ratio reflects the distribution of conjugate subpopulations. This complexity underscores the need for robust analytical techniques and experience, which Raleigh Molecular is well-equipped to provide.
The conjugation process can yield by-products or incomplete reactions, resulting in residues of carrier, drug, and linker compounds. Advanced analytical methods are crucial to monitor the often complex purification steps involved. Sensitive analytical tools are necessary to assess stability, characterize the final product, and ensure batch-to-batch consistency. Raleigh Molecular employs sophisticated analytical techniques to navigate these challenges, ensuring high-quality and consistent outcomes in drug conjugation processes.
Leveraging Raleigh Molecular’s extensive experience in both small molecules and large biopolymers, we cover a broad spectrum including synthesis, analysis, purification, cGMP manufacturing, and aseptic filling, as discussed in the next section. This comprehensive expertise enables us to provide end-to-end solutions in conjugation processes with a focus on quality and efficiency.
Products developed through conjugation at Raleigh Molecular can seamlessly progress to preclinical and early-stage clinical trials thanks to our fully integrated GMP Class 100 Aseptic Filling Manufacturing Facility. Our capabilities encompass sterile filling of liquid-in-vial injectable drugs, aseptic filling and stoppering of liquid prefilled syringes, and aseptic filling and capping of powders. We provide comprehensive regulatory compliance support, including the development, creation, and execution of validation protocols in line with current Guidelines (HPFBI, U.S. FDA, EMA, ICH, WHO) and acceptable formats (prospective, retrospective, and concurrent). This integrated approach ensures quality, safety, and regulatory adherence throughout the development and manufacturing processes.
How Raleigh Molecular Works with Clients
In the typical arrangement, the client specifies the drug and the linkage requirement and provides the carrier molecule. In certain cases, the client may also provide a proprietary drug. Raleigh Molecular obtains or synthesizes the necessary linker, develops the conjugation method, utilizes suitable analytical tools to characterize the product, and collaborates with the client to optimize the drug conjugate for its intended clinical application. This collaborative approach ensures that the conjugation process meets the specific needs and goals of the client’s project.
©2014-2024 Raleigh Molecular Laboratories – All Rights Reserved.